
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
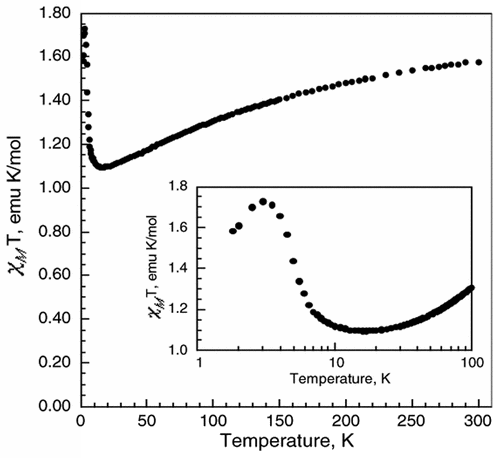
K3Fe(CN)6 reacts with the viologen 1,1′-bis(2,4-dinitrophenyl)-4,4′-bipyridinium dication, (DNP)2+, to form a supramolecular complex, (DNP)3[Fe(CN)6]2·10H2O (1). The crystal structure of 1 reveals that there are two [Fe(CN)6]3– anions within an organic framework of three (DNP)2+ cations with the shortest Fe(III)···Fe(III) distances of ca. 9.8 Å, distances that minimize extensive long-range magnetic exchange coupling interactions between the [Fe(CN)6]3– anions, and, thus, 1 is paramagnetic above ca. 17 K and exhibits weak ferromagnetic coupling between 17 and 3 K and antiferromagnetic coupling between 3 and 1.8 K. The long Fe(III)···Fe(III) distances permit slow spin–spin and slow spin–lattice paramagnetic relaxation, relative to the iron-57 Larmor precession frequency, as is evidenced by the Mössbauer spectra measured between 3 and 60 K; between 85 and 295 K, rapid paramagnetic relaxation is observed. Both the slow spin–spin and slow spin–lattice relaxation are mediated by the organic, π-conjugated viologen cations. The Fe–C distances, the Mössbauer isomer shifts, the temperature dependence of the magnetic susceptibility, and the 3 K magnetization results all indicate the presence of low-spin Fe(III) ions in the [Fe(CN)6]3– anions in 1. There is no unequivocal indication of the presence of any formal electron delocalization or transfer from the [Fe(CN)6]3– anion to the (DNP)2+ cations in the results obtained from X-ray crystallography, magnetic measurements, and Mössbauer spectra. Because of enhancement of the spin–orbit coupling by the heavy-atom or -ion effect, the Fe(III) ions in the [Fe(CN)6]3– anions interact with the (DNP)2+ cations, causing them to fluoresce with increasing intensity upon cooling from 90 to 25 K when excited at 300 nm. The resulting luminescence of the viologen (DNP)2+ cation induced by the [Fe(CN)6]3– anions indicates the presence of significant mixing of the molecular orbitals derived from the [Fe(CN)6]3– anions and the molecular orbitals associated with the (DNP)2+ cations to yield bonding supramolecular orbitals in 1, a mixing that is also observed between 50 and 3 K in the temperature dependence of the isomer shift of 1. | ||||||||
 |
|
|||||||
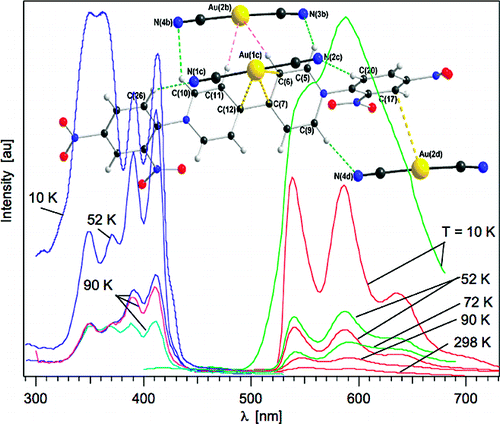
Dicyanoaurate reacts with the organic acceptor molecule, 1,1′-bis-(2,4-dinitrophenyl)-4,4′-bipyridinium, DNP, to form a supramolecular complex with the general formula {[Au(CN)2]2DNP}·4H2O. The complex was characterized by X-ray crystallography, and its photophysical properties were investigated in the solid-state. Although the initial (DNP)Cl2 compound does not show photoluminescence behavior and the dicyanoaurate shows photoluminescence only in the UV range, the resulting supramolecular complex displays two simultaneous, essentially independent, photoluminescence bands in the visible range originating from individual contributions of the DNP unit and the dicyanoaurate dimers. This unusual simultaneous photoluminescence behavior displayed by both the dicyanoaurate donor units and the redox-active 4,4′-bipyridinium acceptor have lifetimes of 0.5 μs and several hundred μs, respectively. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017
